Prevenar 13 and PCV 13 TT
Comparing Prevenar 13 and Vaximune 13 (PCV 13 TT) – Which Pneumococcal Vaccine is Right for Your Child?
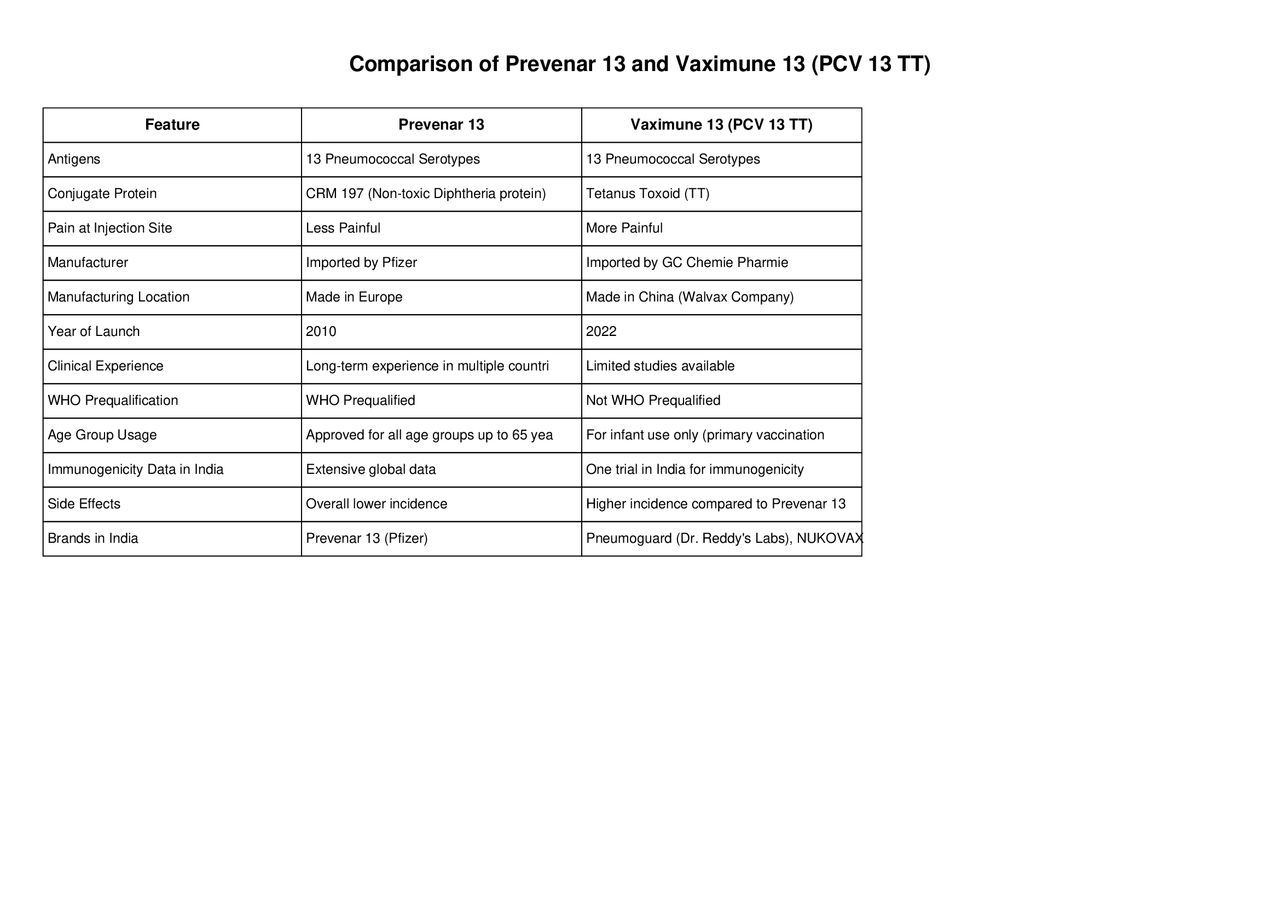
Pneumococcal disease is a serious illness caused by Streptococcus pneumoniae, leading to infections such as pneumonia, meningitis, and sepsis. To protect against these infections, pneumococcal conjugate vaccines (PCVs) are recommended for infants and young children. Two available PCVs offering protection against 13 serotypes of pneumococcus are Prevenar 13 and Vaximune 13 (PCV 13 TT). While both vaccines contain the same antigens, they differ in several key aspects, including conjugate proteins, manufacturer details, clinical experience, and side effects.
Which Vaccine Should You Choose?
Both Prevenar 13 and Vaximune 13 (PCV 13 TT) provide protection against serious pneumococcal infections. However, several factors influence the choice of vaccine:
Prevenar 13 has been in use for over a decade, with extensive global experience and WHO prequalification, making it suitable for infants, children, and even adults up to 65 years.
Vaximune 13 (PCV 13 TT) is a newer vaccine with limited clinical data, currently approved only for infant primary vaccination at 6, 10, and 14 weeks.
Pain and side effects are reportedly lower with Prevenar 13 compared to Vaximune 13.
If long-term safety and WHO prequalification are a priority, Prevenar 13 may be the preferred choice.
Final Thoughts
Pneumococcal vaccination is an essential step in protecting children from life-threatening infections. Parents should consult their pediatrician to determine which vaccine best suits their child’s needs, considering efficacy, safety, and availability. At Vaccine Panda, we prioritize accurate information and trusted vaccines for your child’s health.
For more details or to book your child’s pneumococcal vaccination, visit Vaccine Panda today!
